Soon VAX for children's! Bharat Biotech gets nod to conduct phase 2/3 trials on 2-18 year-olds
Total Views |
New Delhi, May 12: In a very positive development, Hyderabad-based Bharat Biotech got the necessary permission to conduct phase 2/3 clinical trials of Covaxin for children aged 2-18 years. This decision has come amid when experts around the country have opinioned that the third wave of the COVID-19 virus in India can affect children adversely.
According to a news agency, India's drug regulator Subject Expert Committee (SEC) has granted permission to the Bharat Biotech. The drug-making company is going to conduct trials in 525 subjects at various sites including AIIMS, Delhi, AIIMS, Patna, and Meditrina Institute of Medical Sciences, Nagpur.
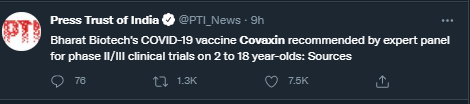
Earlier, Bharat Biotech has sought permission from the SEC to conduct phase II/III clinical trials of Covaxin jabs in children aged 2 to 18 years.
Also Read | Vaccine is only solution to COVID crisis in India: US top health expert Dr Anthony Fauci
The covid-19 drug COVAXIN has been developed jointly by the Bharat Biotech, Indian Council of Medical Research (ICMR), and National Institute of Virology (NIV). It is known as India's homegrown drug against the COVID-19. COVAXIN has shown 78% efficacy against severe COVID-19 disease. It has also effective against 63 covid variants including the Brazil variant.
